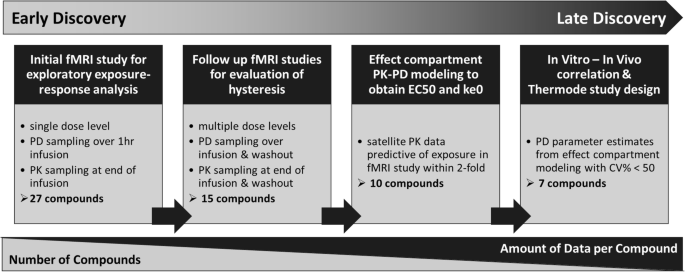
Application of Pharmacokinetic-Pharmacodynamic Modeling to Inform Translation of In Vitro NaV1.7 Inhibition to In Vivo Pharmacological Response in Non-human Primate | SpringerLink

Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
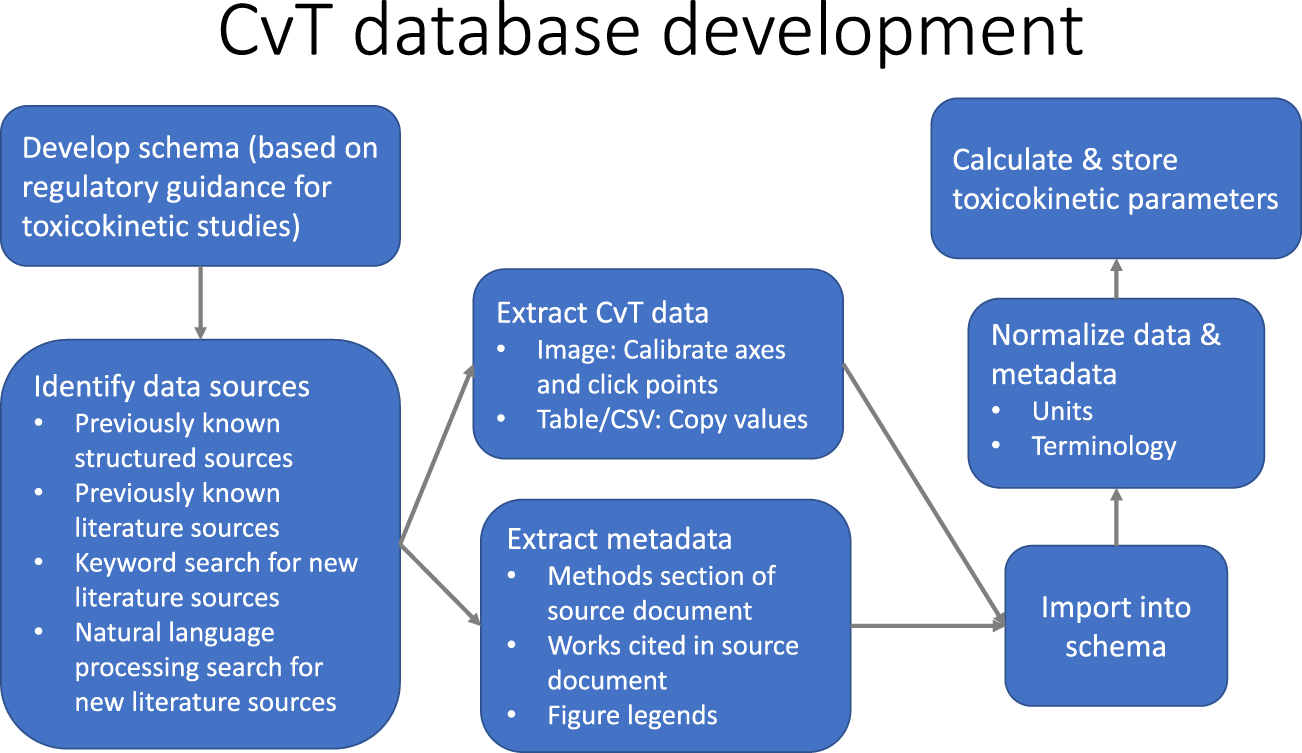
Database of pharmacokinetic time-series data and parameters for 144 environmental chemicals | Scientific Data

Overview of the Clinical Studies Included in the Population PK Analysis | Download Scientific Diagram
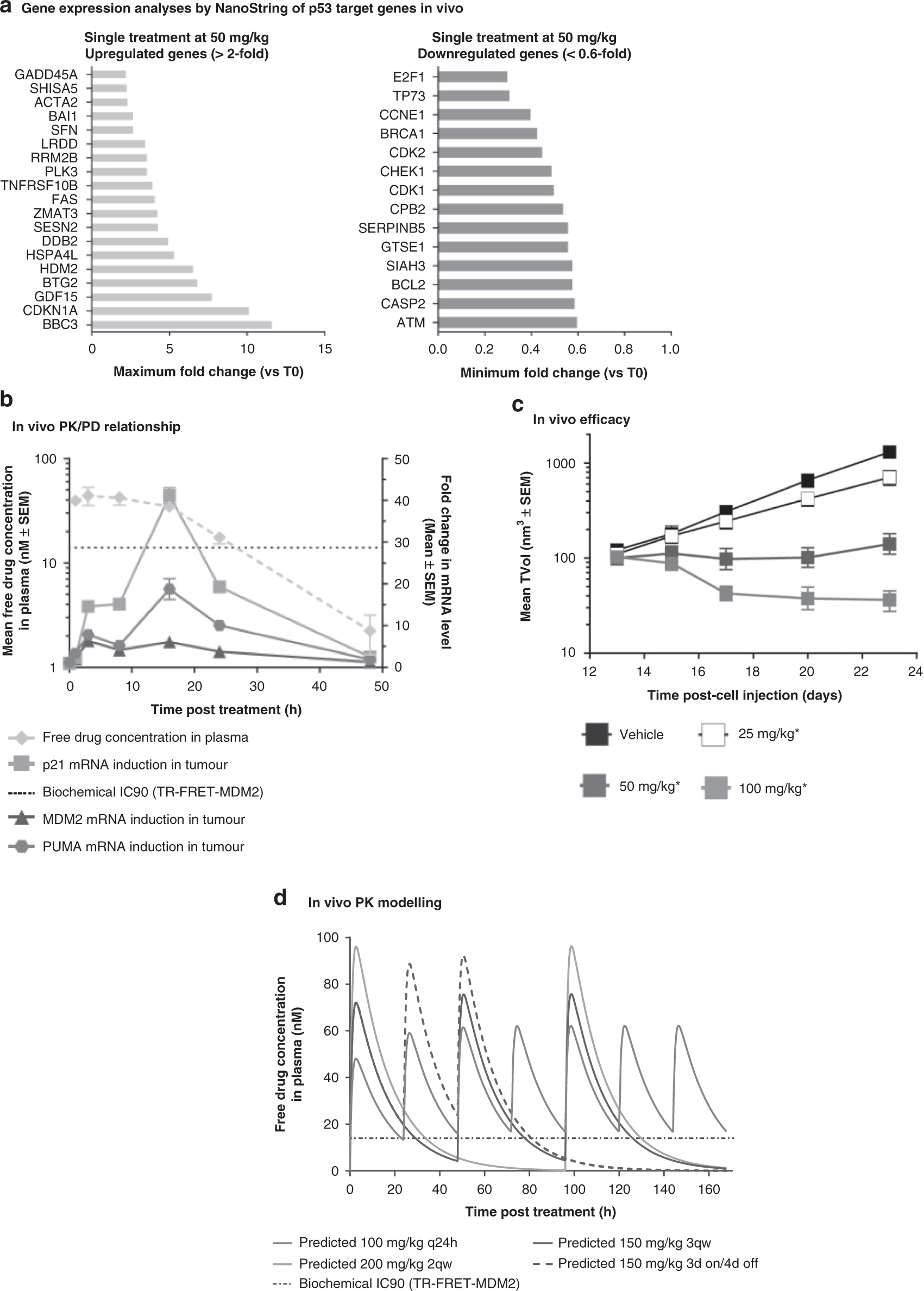
Pharmacokinetic–pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies | British Journal of Cancer

The importance of clinical pharmacokinetic–pharmacodynamic studies in unraveling the determinants of early and late tuberculosis outcomes | International Journal of Pharmacokinetics

Current status and future perspective on preclinical pharmacokinetic and pharmacodynamic (PK/PD) analysis: Survey in Japan pharmaceutical manufacturers association (JPMA) - ScienceDirect
PLOS ONE: Safety, Pharmacokinetic, and Functional Effects of the Nogo-A Monoclonal Antibody in Amyotrophic Lateral Sclerosis: A Randomized, First-In-Human Clinical Trial
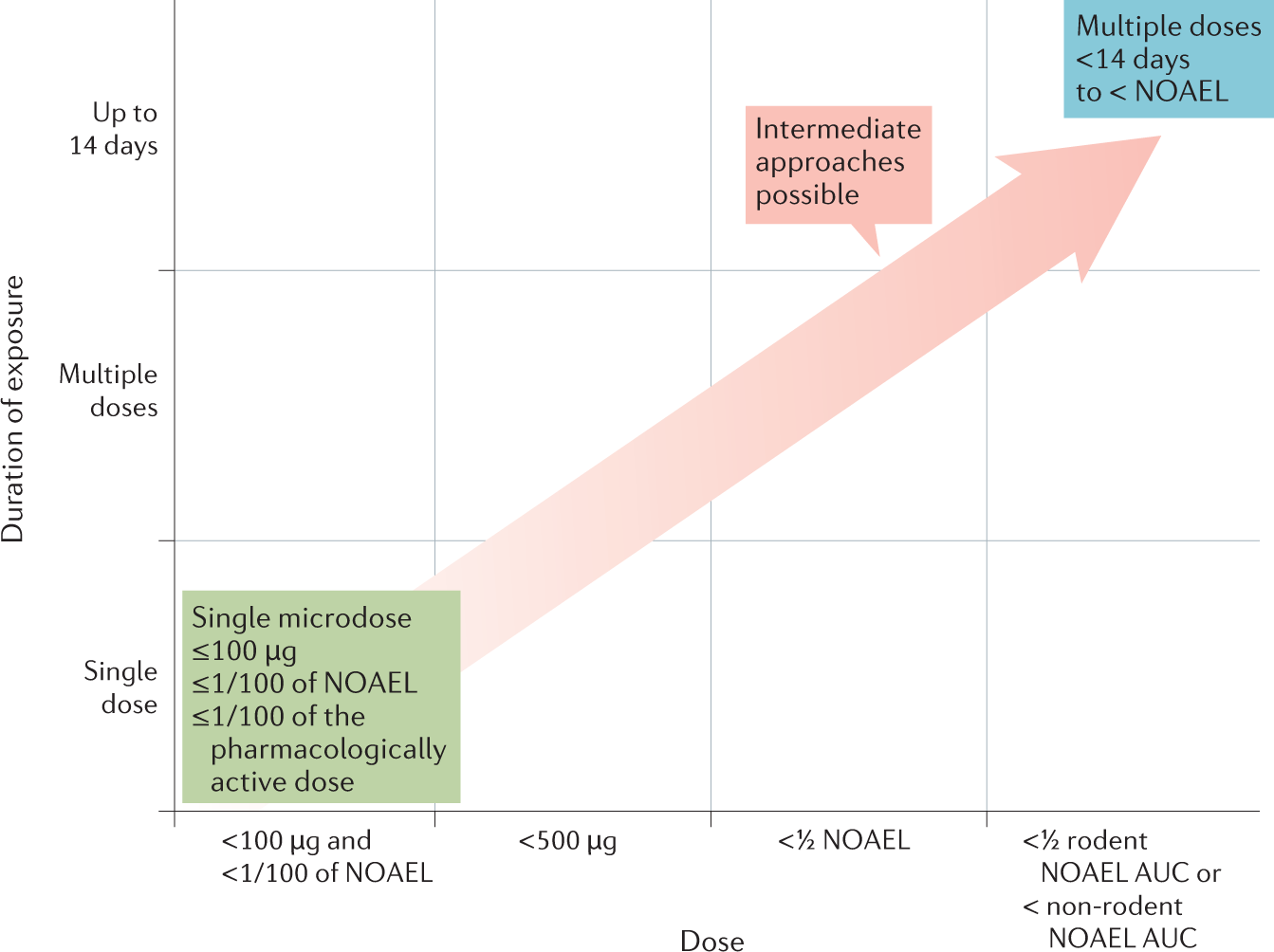
Phase 0/microdosing approaches: time for mainstream application in drug development? | Nature Reviews Drug Discovery

Considerations for Dose Selection and Clinical Pharmacokinetics/Pharmacodynamics for the Development of Antibacterial Agents | Antimicrobial Agents and Chemotherapy

Pharmacokinetic studies in children: recommendations for practice and research | Archives of Disease in Childhood
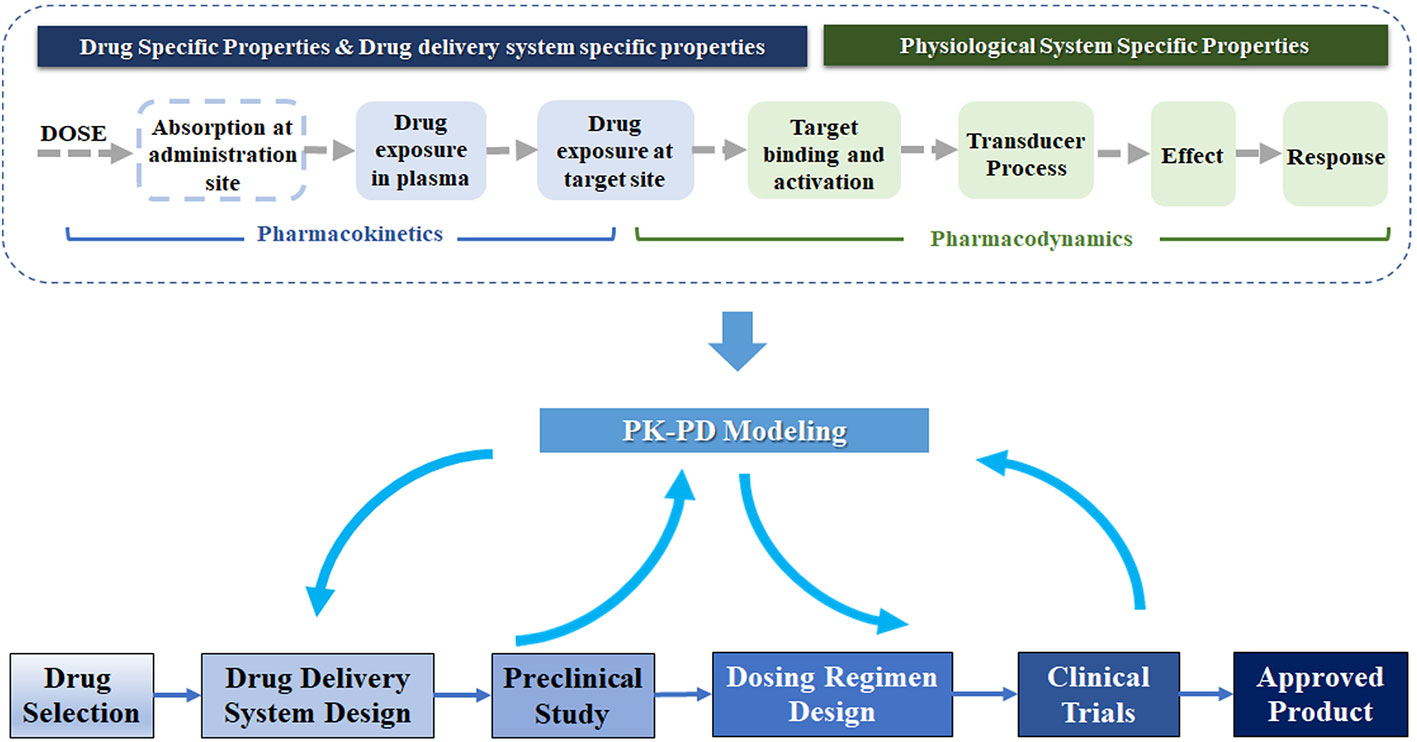
Frontiers | Application of Pharmacokinetic-Pharmacodynamic Modeling in Drug Delivery: Development and Challenges | Pharmacology

Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Pharmacokinetics and Proceedings in Clinical Application of Nucleic Acid Therapeutics: Molecular Therapy