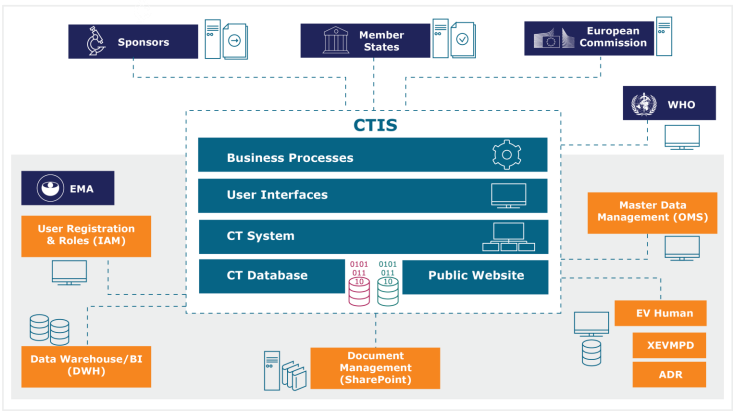
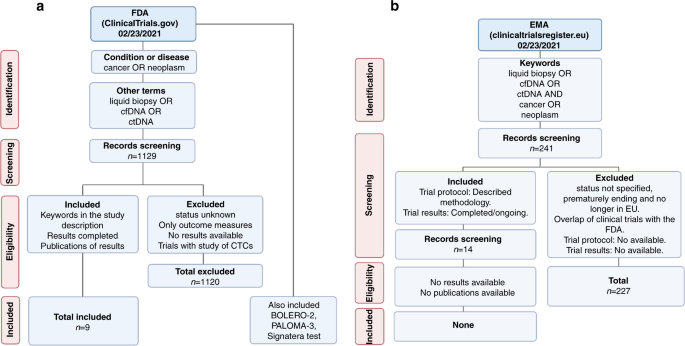
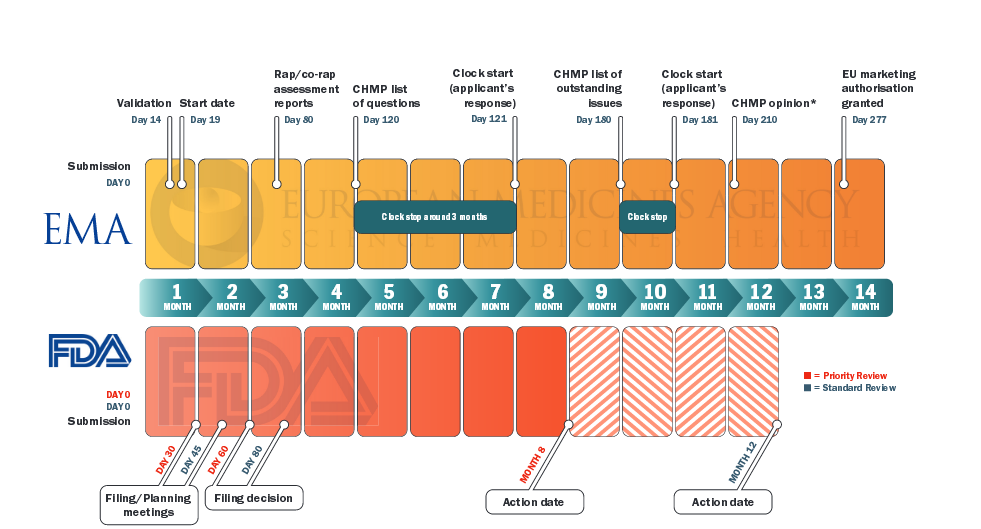
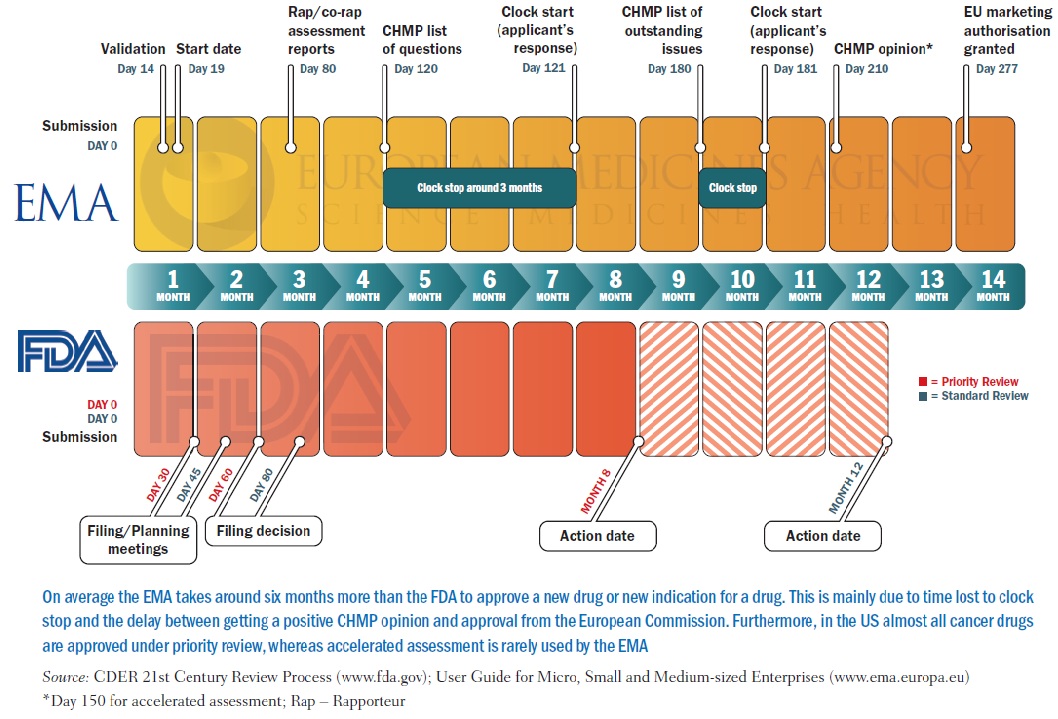
Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy

EU Medicines Agency on Twitter: "In a letter published today, @EU_Commission, #EMA and the Heads of Medicines Agencies remind all sponsors of #ClinicalTrials conducted in the 🇪🇺 to make results of concluded

Assessment of the Regulatory Dialogue Between Pharmaceutical Companies and the European Medicines Agency on the Choice of Noninferiority Margins - Clinical Therapeutics